Innovation Center
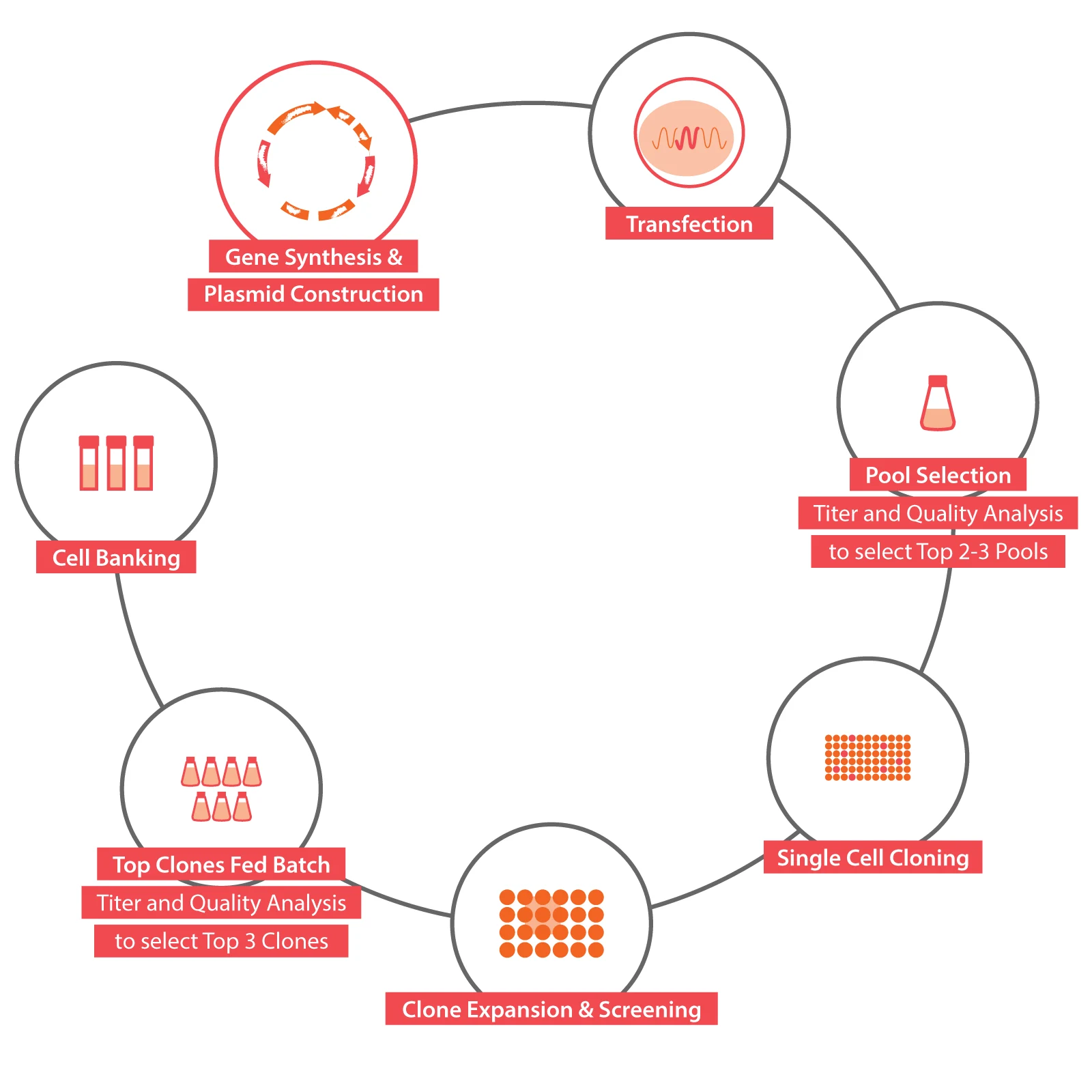
Located in Shanghai Zhangjiang Science City, Chime Biologics Innovation Center, covering an area of over 1500 square meters, focuses on highly efficient cell line development and the state-of-art development of early-stage projects.
It is dedicated to becoming a leading international biopharmaceutical R&D center, complementary to Chime Biologics Wuhan manufacturing facility with its successful experience in multi-country clinical trial applications and new drug launches. Chime Biologics Innovation Center currently provides the services of cell line development, early-stage drug candidate preparation, drug developability studies as well as process development, with the initial capacity to undertake more than 20 biologics projects per year.